Assalamualaikum, Ni hao, Konnichiwa,
Today I would like to share my literature review about this plant. This is one of the elective subject known as "Literature Search (LS)" which is done just by reading several journal. For this, I have read more than 31 sources/journals. Ok actually reading journals, essay, and english is like taking sleeping pills. I really need tonnes and billion of courage to read the journals without fall asleep! Mind you, this is not easy task for me. haha This is like a magic has happen to me when I'm able to finish this within 4 month. But actually, the real time taken to do this is about 1 month.
I would like to note down here or in other word, share with the reader on how I'm going through this.
1st, as usual, I don't know what to do. So, I met my supervisor and she give me the topic. But she did ask me on any plant that do interest me. She gave me the topic Ruta angustifolia.
2nd, just google through google scholar to find journals related to the plant. This is important so that we have general view on what is related to the plant and what is important with this plant. Take note if there is any extraordinary info about the plant.
3rd, do draft.
4th, meet my supervisor. She corrected and improve my draft by giving a lot of comments. hihi~ So now I get clearer picture on how LS is.
5th, start writing. This gonna be the long one, together with the correct format and everything.
6th, submit again and meet my supervisor.
and finally the process of correcting began for about 2-3 times if I'm not mistaken.
Lastly is presentation for 15 minutes.
Then, finish~~~ Horayyyy.
The important thing that i gain from the presentation is about the toxicity of the compound since rutin is being used widely as a supplement. So, it is important for me to stress a lot moreeee on the toxicity part. However, this part is the one that I miss out. I just cover that part around the surface. =(
All the scientific name should be italic but since I copy paste from Microsoft Word to this, the italic has lost. I'm not so 'rajin' to redo that. One more thing, I also did not attach the picture and table since i don't know how to make table here.
Here you go.
1.0 Introduction
5.1 Chemistry
Phenolic --> Flavonoid --> Flavonol --> Rutin --> Quercetin + Rutinose
5.2 Medicinal uses and application
Today I would like to share my literature review about this plant. This is one of the elective subject known as "Literature Search (LS)" which is done just by reading several journal. For this, I have read more than 31 sources/journals. Ok actually reading journals, essay, and english is like taking sleeping pills. I really need tonnes and billion of courage to read the journals without fall asleep! Mind you, this is not easy task for me. haha This is like a magic has happen to me when I'm able to finish this within 4 month. But actually, the real time taken to do this is about 1 month.
I would like to note down here or in other word, share with the reader on how I'm going through this.
1st, as usual, I don't know what to do. So, I met my supervisor and she give me the topic. But she did ask me on any plant that do interest me. She gave me the topic Ruta angustifolia.
2nd, just google through google scholar to find journals related to the plant. This is important so that we have general view on what is related to the plant and what is important with this plant. Take note if there is any extraordinary info about the plant.
3rd, do draft.
4th, meet my supervisor. She corrected and improve my draft by giving a lot of comments. hihi~ So now I get clearer picture on how LS is.
5th, start writing. This gonna be the long one, together with the correct format and everything.
6th, submit again and meet my supervisor.
and finally the process of correcting began for about 2-3 times if I'm not mistaken.
Lastly is presentation for 15 minutes.
Then, finish~~~ Horayyyy.
The important thing that i gain from the presentation is about the toxicity of the compound since rutin is being used widely as a supplement. So, it is important for me to stress a lot moreeee on the toxicity part. However, this part is the one that I miss out. I just cover that part around the surface. =(
All the scientific name should be italic but since I copy paste from Microsoft Word to this, the italic has lost. I'm not so 'rajin' to redo that. One more thing, I also did not attach the picture and table since i don't know how to make table here.
Here you go.
1.0 Introduction
Ruta species is most commonly known as Rue and there are many type of Rue plants available throughout the world. Currently, there are fourteen species of Ruta known as listed in the Plantlist.org which are R. thesioides Fisch, R. buxbaumii Poir, R. chalepensis L., R. coronate Nyman, R. Corsica DC, R. crenulata Burkill, R. erythraea Aitch & Hemsl, R. graveolens L, R. linifolia L, R. macrophylla, R. suaveolens DC, R. montana L, R. patavina L, and R. pedicellata. According to Pollio et al (2008), there are three most diffused species which were poorly differentiated morphologically and probably interchangeably used during antiquity which are Ruta chalepensis L., Ruta graveolens L., and Ruta montana (L.) L.
The geographical distribution of the plant is known to be native to the Mediterranean region. However, currently the plant has been distributed at the other countries such as Canary Islands, Ehiopia, Arabia, South America, Mexico, and West Indian islands.
Flavonoid is important in Ruta plant for self-protection. The compound works by promoting physiological survival of the plant by protecting from pathogenic microorganisms and UV radiations. The compounds also used for pigmentation of flowers, fruits and seeds in order to attract pollinators and seed dispersers, morphogenesis, sex-determination, and in plant-microbe interactions. There are few types of flavonoids available in the Ruta species which are rutin, quercetin, apigenin, luteolin, eriodictyol, and naringenin (Proestos et al, 2005).
Besides than its importance for the plant itself, this natural polyphenolic molecules of plant origin are well-known for the human benefit as an anti-oxidant, anti-inflammatory, anti-carcinogenic, anti-allergic, anti-viral, and many more (Coman et al, 2012).
2.0 Botanical description of Ruta angustifolia (L) Pers.
Nowadays, the plant is popular with the name of Ruta angustifolia [L.] Pers., but the plant is actually has been known previously as Ruta chalepensis auct, Ruta graveolens auct, Ruta graveolens L. var. angustifolia (Pers.) Hook. f., and Ruta chalepensis L. var. angustifolia (L.) Backer as stated in the Book of World Spice Plants : Economic Usage, Botany, Taxonomy (2005). R. angustifolia is also known as fringed rue, Egyptian rue, or Syrian rue. Plant name will be different based on the location whereby the Chinese community called it as Aruda or Garuda (currently known), the Malaysian community called it as Sadal, while the Indonesian called it as Godong minggu or Inggu.
Details about the botanical characteristics of the R. angustifolia should be familiarize by the researcher in order to prevent misidentification with other similar type of Ruta species. R. angustifolia is a perennial herb, shrubs, woody at the base, 0.3 to 1.5 metre tall with average adult height 0.4 metre, yellow color of flowers, sharp fruit lobe tops, bitter taste of leaves, and have strong fetid smell (Kannan & Babu, 2012). R. angustifolia is determined by its leaves arranged spirally, bluish-green, emit a powerful odour, crenate, narrow or oval leaves, 2–3 times divided into segments oblong and ciliate-fringed sepals. It also has ciliate or fringed petals (Pollio et al, 2008; Kannan & Babu, 2012; Haddouchi et al, 2013). A better illustration can be referred to the Figure 1 and Figure 2. In general, R. angustifolia taxonomy can be simplified as in the Table 2 whereby the plant has the characteristics of a a dicotyledons plant, vascular, propagate by seed, and flowering. The plant is able to grow on any soil but a well-drained calcareous clayey soil and fairly dry conditions in partial shade is more preferable.
Microscopic characters of the R. angustifolia (synonym : R. chalepensis) also has been studied by Kannan & Babu (2012) and Mokhtar (1987). Brief summary of the microscopic characteristics can be seen in the Table 1. Stem transection shows a nearly pentagonal outline with blunted corner. The arrangement started from outer epidermis followed by hypodermis, cortex, vascular zone and central pith. Epidermis and hypodermis are single layered. Cortex is differentiated in to outer few layers of chlorenchyma and inner parenchyma. Chlorenchyma is loosely arranged as aerenchyma with lot of air spaces. Parenchyma is normal with intercellular spaces. Pericycle is made of patches of lignified fibers. Lumen of fibers is narrow and represented as a dot. Phloem and xylem shows usual elements. Pith is large, parenchymatous and undifferentiated. Minute starch grains are observed in parenchyma cells of cortex, especially more in chlorenchyma region than other cells. Pith cells do not show any starch grains. Calcium oxalates are observed in parenchyma cells of cortex and pith. It is abundant and observed plenty in each and every field observed. Furthermore, the plant leaf shows 3-5 vascular bundles in an arc. Midrib and lamina showed a typical dicotyledon structure with single layered epidermis, double layered palisade cells and loosely arranged spongy cells. Figure 3 and Figure 4 can be seen for the illustration (Kannan & Babu, 2012; Mokhtar, 1987).
Since the plant has been discovered and used traditionally for a long time ago, there is already commercially available product of R. angustifolia. A sample of chopped pieces or the coarsely powdered aerial parts of R. angustifolia (synonym : R. chalepensis) can be bought in bulk. The sample has a strong aromatic and fetid odour with green to dark green in colour. Almost in all the supplies contain stem, leaves, inflorescence, flowers, and fruits parts of the R. angustifolia. Stem pieces are up to 1 cm in thickness while the pith is mostly hollow in thicker pieces. (Kannan & Babu, 2012).
3.0 Medicinal properties of Ruta angustifolia (L) Pers.
R. angustifolia can be classify as one of the important medicinal plant. World Health Organisation (2008) has defined medicinal plants as plants that contain properties or compounds that can be used for therapeutic purposes or those that synthesize metabolites to produce useful drug. A large proportion of such medicinal compounds have been discovered with the aid of ethno-botanical knowledge of their traditional uses. Medicinal properties derived from plants can come from many different parts of a plant including leaves, roots, bark, fruit, seeds, and flowers. The different parts of plants can contain different active ingredients within one plant. Thus, one part of the plant could be toxic while another portion of the same plant could be harmless. Medicinal parts of Ruta species used usually all parts which are the leaves, young stem, root, and flowers (Stuart, 2005; Gupta et al, 2014; Madhu, 2014).
3.1 Traditional uses
Ruta species has been used traditionally for various conditions such as fever, influenza, cough (plant decoction), headaches (plant decoction), chest pain, hiccup, intestinal worms, ulcers, inflammation, contraceptive, hypertension, hysteria, and relieve symptoms of hangover (Stuart, 2005). Other than that, it is also have been used topically for ear aches (heated leaves), skin antiseptic, insect repellent, and as a poultice against rheumatic pain (leaves and seeds boiled with oil) (Mokhtar Saleem, 1987). According to Emam et al (2009), R. angustifolia (synonym : R. chalepensis) is widely used even as an anti-spasmodic (flowering branches), snake bites treatment (essential oil of leaves), molluscicidal activity, larvicidal activity and repellent activity. The plant is among the most-used genera in contemporary Italian traditional medicine, economic botany, and folk life.
The plant has also been used internally by firstly soaking the leaves, root, and seed in wine or mixing the leaves, root, and seed with honey or its derivatives before administration (Pollio et al, 2008).
3.2 Biological and Pharmacological Activities
A study done by Haddouchi et al (2013) on anti-fungal activity reveals that R. angustifolia oil has a powerful antifungal activity against filamentous fungi. The result shows that Aspergillus fumigatus and Cladosporium herbarum are the most sensitive strains to these oils with minimum inhibitory concentration (MIC) values of less than 3.5 ug/ml while the other species require higher MIC value. Higher MIC value means increase amount of R. angustifolia oil are required to exhibit the anti-fungal activity. Table 3 below shows in-vitro MIC values which was measured in the unit of ug/ml of R. angustifolia essential oils against yeast and filamentous fungi and the diameter of disc inhibition (diameter disc 6mm included).
The study from Haddouchi et al (2013) also showed that by the oil of R. angustifolia shows no antibacterial activity on all strains being tested. The bacteria used in this research are Enterobacter cloacea, Enterococcus faecalis, Acinetobacter baumanii, Bacillus cereus, Escherichia coli, Citrobacter freimdoo. Pseudomonas aeruginosa, Klebsielle pneumaniae, Lysteria monocytogenes, Proteus mirabilis, Staphylococcus aureus, and Salmonella typhi.
Herb and drug interaction is also a part of concern in the pharmacological activities. Since the elderly group usually consume the herbs, they usually associated with certain types of diseases and for sure is on multiple medications. This can cause herb and drug interaction between the herbs and the drug since the herb contain coumarin. According to Stuart (2005), there is potential interaction with anti-hypertensive and photosensitizing medications. Taking photosensitizing drug with the herbs will increase sensitivity towards sunlight which eventually could increase the chances of sunburn and blistering or rashes on the exposed skin area. Example of photosensitizing drugs are Amitriptyline, Trioxsalen, and several types of antibiotics such as Ciprofloxacin, Ofloxacin, Levofloxacin, Gatifloxacin, Trimethoprim/Sulfamethoxazole, and Tetracycline (WebMd, 2009). However, there is no article can be found which explain specifically about the interaction in details.
4.0 Phytochemical of Ruta angustifolia (L.) Pers
According to Mosby’s Medical Dictionary 8th edition (2009), phytochemical means the active chemical components, or constituents, present in a plant that account for its medicinal properties. While essential oil can be defined by a dictionary as a volatile oil which generally extracted from plants using a steam distillation process, have the characteristic odour or flavour of the plant, and are used to make perfumes and flavouring.
Flavonoids is a water soluble polyphenolic molecules containing 15 carbon atoms, belong to the polyphenol family and can be visualized as two benzene rings which are joined together with a short three carbon chain. According to Higdon et al (2008) from Oregon State University, flavonoids can be divided into few subclass which are anthocyanidins, flavanols, flavanones, flavonols, flavones, and isoflavones. Each subclass will have their own nomenclature whereby the nomenclature is based on the substituents position. Examples of subclass of flavonols are kaempferol, morin, rutin, myricetin, quercetin, quercetrin, myricitrin, spirenoside, galangin, robinin, kaempferide, fisetin, and rhamnetin. Flavonoids is responsible for the natural colorant of fruits, vegetables and herbs. Green tea, black tea, apple, and citrus fruits are rich in flavonoid.
There are many types of chemical compounds exist in the oil of R. angustifolia according to Wikiherb.info and Sciencedirect.com which have been classified as in the Table 4.
According to Haddouchi et al (2013), the essential oil class of R. angustifolia has six types which constitutes 96.47% of the class which are 2-ketones representing 95.63% and aldehydes representing 0.84%. The most dominant compound is 2-Undecanone constitutes 82.46%. The other compounds which exists in the plant are 2-Decanone representing 10.03%, 2-dodecanone representing 1.51%, 2-Nonanone representing 0.96%, 2-tridecanone representing 0.67%, and Nonanal representing 0.84%.
5.0 Rutin
It is important to know that there are many synonym names for rutin which are rutinum, flavonol glyscoside, 3-rahmnosyl-glucosyl quercetin, quercetin-3-rhamnosyl-glucosyl, rutoside, and phytomelin. There are many other names used for rutin which has been listed in the PubChem Compound Database (2015). The most popular one is quercetin-3-rutinoside which is also known as Vitamin P (Natural Product catalog, n.d.). However, for WHO, they called it as 3-rhamnoglucosie of 5,7,3',4'-tetrahydroxy-flavonol.
Rutin is present naturally in fruit, vegetables, and beverages such as tea and wine (Gupta et al, 2014). Red wine contains high levels of rutin. Major sources of rutin include Fagopyrum esculentum (buckwheat), Styphnolobium japonicum (Japanese pagoda tree), and Eucalyptus macrorhyncha (red stringybark) (Drugbank, 2013). Other plant species of that contain rutin are lime tree flowers, elder flowers, hawthorn, St. John's Wort, Ginkgo biloba, tobacco, and Ruta species. (WebMD.com, n.d.; Drugbank, 2013).
This paper will be focusing more on Ruta angustifolia (L) Pers specifically about Rutin.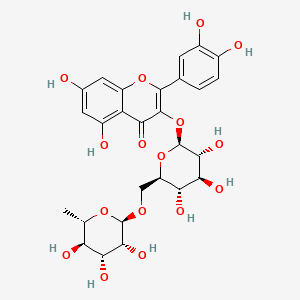 |
| Rutin chemical structure |
Each of the plant have various types of compounds and one of the important compound is flavonoid rutin. There are various factors affecting biosynthesis and accumulation of flavonoid and essential oil contents in Ruta species which are geographical location, time of sowing, type of fertilizers (Soheir et al, 2008), type of plant organ, climate, genotype, chemo-types, and extraction procedure (Haddouchi et al, 2013). For example, Ruta plant growing in shade will have different properties from another pushing sunlight. Therefore, it is important to take note that by varying the variables will affect the flavonoid and essential oil content in the plant.
Rutin biosynthesis and content in the plant will vary under different condition and situation such as drought/water, salinity, presence of sucrose content, temperature, light, plant hormones, and fertilizers. There are few conditions that cause increase in rutin content. First is the presence of UV-radiations cause stimulation of the activity of enzymes involve in phenyl-propanoid pathway causing an increase of rutin content in the plant. Next, the rutin content in the seedlings of Dimorphandra mollis increased when exposed to stress condition. The increase of rutin shows their role in protecting tissues against oxidative damage. Another example is the usage of an excessive fertilizer cause an increase in plant vigour which negatively influence flavonoid content in grape berry. Next, the accumulation of rutin increases with increased in sucrose content in Arabidopsis, buckwheat seedlings along with the expression of flavonoid genes. Meanwhile, the presence of methyl jasmonate showed an inhibitory effect on rutin content in Cucurbita and Buckwheat. Methyl jasmonate is a volatile organic compound used in plant defence system and other plant development (Gupta et al, 2014).
Besides extracting the rutin content from the natural sources, the compound can also be produced synthetically. General flow of flavonoid biosynthesis pathways can be seen as in Figure 6. The process involves pentose phosphate pathway (PPP) and Shikimate pathway. Initially, glucose is converted to erythrose 4-phosphate by PPP and then undergo Shikimate pathway to form aromatic amino acid, and finally followed by phenyl-propanoids, flavonoids, and complex flavonoids. Figure 7 shows the flavonol biosynthesis in more details starting from the amino acid, phenylalanine. It involves phenylpropanoid pathway which transforms phenylalanine to coumaroyl-CoA. It is initiated by the enzymatic step catalysed by chalcone synthase (CHS), resulting in the yellow coloured chalcone. Precursor for rutin synthesis are malonyl-CoA and p-coumaroyl-CoA which are derived from carbohydrate metabolism and phenylpropanoid pathway. (Falcone Ferreyra et. al, 2012; Gupta et al, 2014).
After knowing its synthesis, it is also important to be familiar with the chemical structure of the rutin. Rutin is phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety and is a large family of low molecular weight polyphenolic secondary metabolites. Generally, the structure of rutin is the same as the flavonoids family which has two phenyl rings, C6-C3-C6. This flavonol glycoside comprised of the quercetin and the disaccharide rutinose (rhamnose and glucose) with molecular formula are C27H30O16 and 610.5175 g/mol molecular weight. These description can be seen in diagram as in Figure 5 (Gupta et al, 2014). Pure rutin powder appear white to light yellow crystal powder (guidechem.com) or yellow-green colored needle-shaped crystal (phytochemicals.info). The powder has very high melting and boiling point which is 125oC and 195oC respectively. The powder is stable in closed containers at room temperature under normal storage and handling conditions. It is also water soluble with 125mg/L solubility (Guidechem.com, Drugbank, Pubchem). In term of heat stability, flavonoids are heat stable while phenolics are less stable and thus may have structural changes when expose to high heat (Motamed et al, 2014).
Rutin is a structural derivate of dissacharide, quercetin, 1-benzopyran, and phenylpropanoid (CHEBI Team). Rutin class can be classified into simpler form as the following flow :
5.2 Medicinal uses and application
Rutin has broad range of medicinal application in human. The health-promoting effects of rutin may be related to interactions with key enzymes, signaling cascades involving cytokines and transcription factors, or antioxidant systems (Webmd.com). Rutin have shown a wide range of biological activities such as anti-oxidant, anti-bacterial, anti-viral, anti-hyperglycemic, anti-cancer, anti-fungal, reduce triacylglycerol levels, insecticides properties, capillary stabilizing agents or vasoprotectives, and hepatoprotective (Stuart, 2005; Pubchem; Madhu, 2014; Gupta et al, 2014; Journal of Pharmacy & Pharmacology, 2003).
Rutin exhibited significant anti-oxidant activity in a liposomal model reaction. Rutin can suppress glycation of aminoguanidine substrate. G-rutin is a water soluble glucose derivative of rutin, derived by enzymatic transglycosylation, a potent glycation inhibitor especially in kidney problem patient, exhibit antioxidant capacity in-vivo by inhibiting DNA and protein oxidation. The antioxidant activity of rutin corresponds to a strong affinity to suppress the formation of both initial and advanced stages of Maillard reaction in tissue protein sources. Rutin showed similar significant protection against peroxyl
radical induced lipid oxidation compared to the control sample not containing
antioxidant. Bioflavonoids rutin reduce the ROS generation and have both free
radical scavenging and metal ion chelation (Nagasawa et al, 2003). According to
Journal of Pharmacy & Pharmacology (2003), Rutin derivatives have superoxide
radical scavenging activity and hydroxyl radical scavenging activity. The rate
constant of hydroxyl radical scavenging of rutin seems to increase with the
number of hydroxyethyl groups present (Haenen et al, 1993). Rutin increases
intracellular ascorbic acid levels, decreases capillary permeability and
fragility, scavenges oxidants and free radicals, inhibits destruction of bones,
and lowers the risk of heart diseases. Epidemiological studies have illustrated
that heart diseases are inversely related to the flavonoid intake. Rutin also protect
cells against the damaging effects of reactive oxygen species by maintaining
ROS balance. An imbalance between antioxidants and reactive oxygen species may
result in oxidative stress, leading to cellular damage. Oxidative stress has
been linked to many other diseases such as cancer, aging, atherosclerosis,
ischemic injury, inflammation, and neurodegenerative diseases.
Rutin also shows protective effect from the carcinogen-induced DNA damage in the research done by Webster et al (1996) as an anti-cancer. Administration of hepatocarcinogens aflatoxin B1 and N-nitrosodimethylamine to rats caused single-strand breaks in nuclear DNA. The protection against DNA damage by the dietary rutin intake was found to be because of the induction of repair enzymes polymerase, DNA polymerase β and DNA ligase. The presence of rutin causes significantly reduced the appearance of breaks. Since DNA damage and inefficient repair are expected to initiate the process of carcinogenesis, modulation by rutin of these parameters has shown the protective role against carcinogenesis induced by chemical carcinogens. Rutin can also be used to prevent mucositis which is a side effect of anti-cancer treatment (WebMD.com) and to prevent lipid peroxidation because the process will cause cancer (Motamed et al, 2014).
Antifungal activity has been described in detail in part 3.2 above.
Since the people believe that rutin can strengthen blood vessels, they used it for varicose veins, internal bleeding, hemorrhoids, and strokes prevention (WebMD.com). According to Jasuja et al (2012), rutin is a potent thrombotic inhibitor of protein disulfide isomerase (PDI) reductase in-vitro which will inhibit aggregation of human and mouse platelets and endothelial cell-mediated fibrin generation in human endothelial cells. This eventually blocks platelet accumulation and fibrin formation. Dietary rutin acts by binding to the blood vessel wall which may exhibit antithrombotic activity but was not be detected in the plasma. PDI is the prototypical member of an extended family of oxidoreductases. Platelets are a rich source of extracellular PDI and are expressing this protein on their surface and secreting PDI in response to thrombin stimulation. However, the best part of rutin is the activity of rutin in antithrombotic can be completely reversed by infusion of recombinant PDI.
Another study has been done by Coman et al (2012) which study the rutin biological properties as an anti-hyperglycemic. From the research, it shows that rutin has the ability to positively influence insulin activity and insulin resistance in type 2 diabetic rats by decreasing glycaemia and lipidaemia, reduce formation of glycated haemoglobin, improves serum insulin concentrations and liver glycogen content, and hexokinase activities. Rutin also effective in increasing the expression of the receptor PPAR-γ, leading to improved muscle insulin sensitivity and insulin signalling by increasing insulin-stimulated glucose receptor 4 (GLUT4) receptor activity. GLUT4 is the receptor that accounts for most of the insulin-stimulated glucose uptake in muscles and adipose tissue cells. The antioxidant property of the natural compounds may be acting synergistically with their hypoglycaemic activity in exerting an overall anti-diabetic action.
Another studies have shown that flavonoids in dietary tea drink will prevent the oxidation of low-density lipoprotein (LDL) and lower the blood levels of cholesterol and triglycerides and thereby reducing the risk for the development of atherosclerosis. Iso-flavones can also help to reduce blood cholesterol level with extra benefit of osteoporosis prevention and ease the menopausal symptoms (Phytochemical.com).
Rutin has been proven to be an effective anti-bacterial by the Orhan et al (2010). The mechanism is believed to involve an inactivation of cellular enzymes, which depended on the rate of penetration of the substance in to the cell or caused by membrane permeability changes, increased membrane permeability where rutin may disrupt membranes and cause a loss of cellular integrity and eventual cell death (Hayriye, 2011).
Rutin can also be used as anti-viral. Rutin had shown significant inhibitory activity against the RNA virus Parainfluenza virus 3 (PI-3), which was comparable to anti-viral Oseltamivir (0.16–0.012 mg/ml) (Orhan et al, 2010). The study also being supported by Deca et al (1987) and Kumar & Pandey (2013) which shows the rutin anti-viral activity against Parainfluenza virus, influenza virus, and Potato virus. Inhibition of viral polymerase and binding of viral nucleic acid or viral capsid proteins have been the proposed antiviral mechanisms of action.
Other medicinal uses of rutin are for treatment of osteoarthritis when use in combination with the proteins trypsin and bromelain. Rutin can also be used to heal swelling in the arm after breast surgery (post-surgical lymphedema) (WebMD.com).
There are already many type of brands containing rutin as active ingredients available in the market. The product mostly has single ingredient rutin only but there are also combination of rutin with ascorbic acid, selenium, and buckwheat. Name of products available in the market are classified in the Table 5 and Table 6. For general information, Rutin has become China's important export of medicinal plant extracts of raw materials products. Rutin also can be used for medicine, health food, or cosmetics products (Gupta et al, 2014).
A table of biological and pharmacological properties, mechanism of action, and tested organism/ cell have been summarized in the Table 7.
5.3 Side effects and adverse effect
Unfortunately, there is side effects that can be identified when consuming Rutin drug. The unwanted side effects are hives, difficulty breathing, swelling of face, lips, tongue, or throat. Other more common side effects of Rutin capsule are blurred vision, dizziness, dull ache or feeling of pressure or heaviness in legs, fluid accumulation in the knee, headache, itching skin near damaged veins, pounding in the ears, red, scaling, or cursed skin, irregular heartbeat, and swollen feet and ankle, or stomach upset (WebMD.com; Drugs.com).
However, Rutin is likely safe when taken by mouth in amounts found in fruits and vegetables (WebMD.com). However, there is also potential moderate herb-drug interaction of rutin with warfarin (Drugs.com) since rutin may increase the effect of anti-thrombotic medication.
5.4 Toxicity data
A research article written by Motamed et al (2014) and Stuart (2005) have showed that there is evidence of toxicity and carcinogenicity of artificial rutin as anti-oxidants when consumed by the human. The phytotoxic constituents responsible for the toxicity are 5-methoxypsoralen (5-MOP) and quinolone alkaloid graveoline show embryotoxic and mutagenic effect. These can also cause serious photo-dermatitis and contact dermatitis. Fortunately, the research shows that the natural anti-oxidant is much more safer than the use of synthetic rutin.
Comparing the artificial rutin with the natural extract, there are some supportive information regarding the toxicity of rutin. By assuming the water-soluble rutin will be presence in the infusion and extracts of R. angustifolia, an experiment was done to study the toxicity effect. Dried leaf infusions of R. angustifolia (synonym : R. chalepensis) were found to cause perinatal changes in mice, at daily doses of 0.16, 0.80 and 1.60 g/kg, administered orally from 1 to 14 days post coitum, showing embryotoxic effects (Zeichen et. al., 2000). In another study, acute (24-h) and chronic (90-day) oral toxicity studies on the ethanolic extracts of R. angustifolia (synonym : R. chalepensis) aerial parts which was carried out in mice had revealed a significant fall in RBC level in treated animals (Shah et. al., 1990). The LD50 of 10% ethanolic extract when administered orally and intra-peritoneally to the animal is 6400mg/kg or 563mg/kg respectively (Mokhtar Saleem, 1987).
However, a research done by Jasuja et al (2012) on the dietary rutin has showed that there is no toxicity in cultured endothelial cells for at least 72h at concentration as high as 100uM. Chronic administration at concentration as high as 3000mg/kg also showed that there is no significant toxicity in animal studies. Dietary rutin are well tolerated and there is a feature of rutin which is the same glycosidic linkage causing it to has lack of toxicity that is required for inhibition of PDI activity impairs cell permeability.
6.0 Conclusion
Ruta angustifolia extract is a good alternatives potential for the source of rutin in management of various types of disease condition especially when there is increasing emergence of resistance worldwide and the usage of synthetic anti-oxidant possess high risk of side effect. Nowadays, there is a lot of cases of toxicity and carcinogenicity of synthetic additives has been reported. Therefore, it is crucial for most of the food companies to find the naturally occurring antimicrobial compounds suitable for use in food such as from Ruta extracts. Rutin extract has no risk of side effects compared to the pure rutin compound and therefore more study is required to make it more convincing. Rutin extract from R. angustifolia does exhibit many important health benefit such as anti-oxidant or free radical scavenging power, anti-cancer, anti-fungal, anti-thrombotic, anti-diabetic, anti-bacterial, and anti-viral.
My next plan is I would like to do book review and share my thought and knowledge in this blog too. I wish I'm able to do so. Please pray for me. hihi
Enjoy reading to the last..:p That's all.
Jaane~


Comments